Laboratories play a crucial role in identifying early active cases of Tuberculosis (TB), which is a key to eliminating the disease worldwide. In order to meet WHO’s goals for prevention of TB transmission and improvement of patient care, rapid testing of TB suspects with sensitive molecular diagnostic tests is recommended by the Global Laboratory Initiative (GLI) and experts from the European Tuberculosis Laboratory Initiative (WHO European Region). Both global expert groups recommend the use of Cepheid’s Xpert MTB/RIF as a frontline test, rather than smear microscopy or line probe assays in conjunction with culture-based methods for drug susceptibility testing.1,2
In its publication, GLI outlined a preferred algorithm for promoting universal patient access to rapid testing for TB and rifampicin resistance.1 Read more about the GLI model by clicking here.
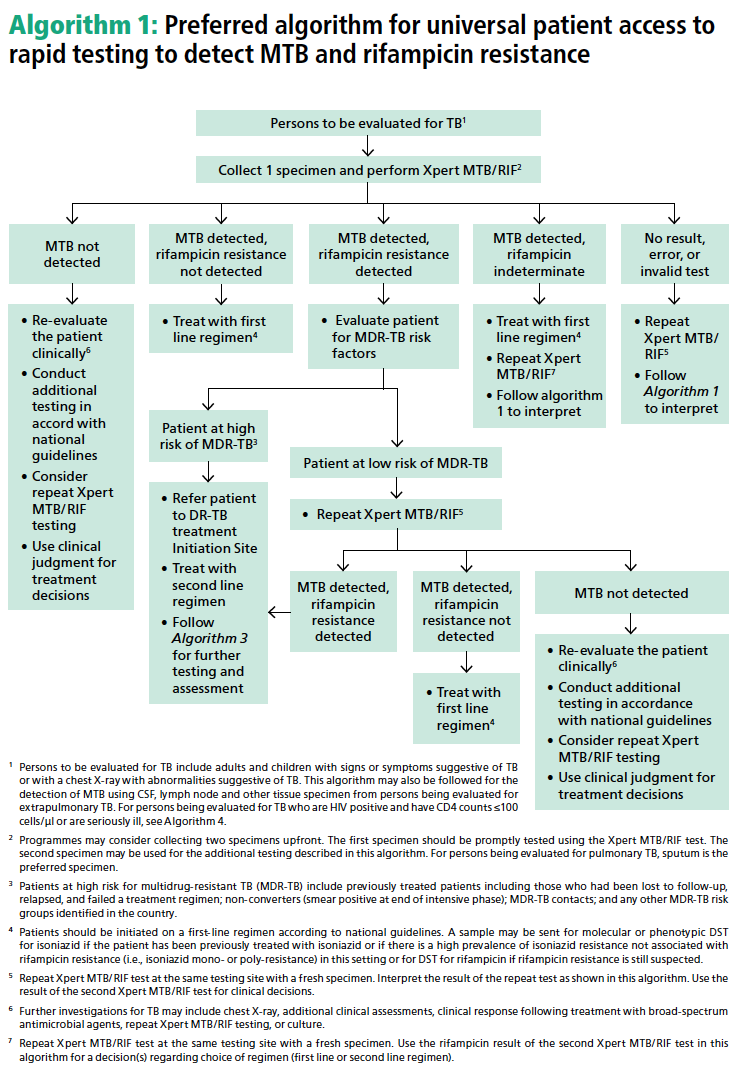
1. Global Laboratory Initiative. GLI model TB diagnostic algorithms. Accessed Mar 2017. http://www.stoptb.org/wg/gli/assets/documents/GLI_algorithms.pdf
2. European Tuberculosis Laboratory Initiative. Algorithm for laboratory diagnosis and treatment-monitoring of pulmonary tuberculosis and drug-resistant tuberculosis using state-of-the-art rapid molecular diagnostic technologies. Accessed Mar 2017. http://www.euro.who.int/__data/assets/pdf_file/0006/333960/ELI-Algorithm.pdf








